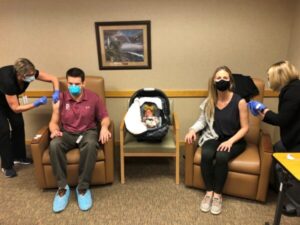
Initial doses of the COVID-19 vaccine are being distributed to Sleepy Eye Medical Center staff this week. Some of the first to receive the vaccine include SEMC providers and hospital and clinic nurses.
“We will vaccinate additional employees in the coming weeks as shipments arrive,” said Kevin Sellheim, SEMC Administrator. “There are limited doses in these initial shipments, and it will take weeks to vaccinate staff. It could be months before it’s readily available for the general public.”
Vaccination is taking place in a phased approach across the state, with healthcare workers, long-term care residents and workers, and emergency medical services personnel included in phase one. Prioritization groups have been established to maximize immediate health benefits, reduce death and serious illness, and minimize the harm created by COVID-19. SEMC has been working with the South Central Healthcare Coalition and other partners to help ensure that the vaccine gets to those in priority populations.
“Moving from one phase to the next will be determined by vaccine supply and uptake,” said Sellheim, “So we can’t give firm dates for when one phase will end and another will begin.”
SEMC providers support the vaccine and believe vaccination is one of the best ways to protect yourself and others around you from COVID-19.
“The vaccine is going to be the most effective tool that we have for controlling this pandemic from a public health standpoint. If the vaccine can successfully prevent severe disease, the impact of COVID-19 will diminish significantly and allow a return to normalcy,” said Dr. Adam Armbruster, SEMC.
In addition, SEMC providers assert that the vaccine is safe.
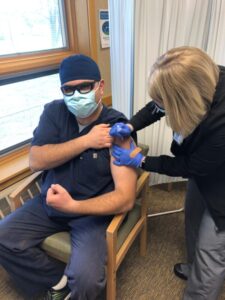
“I truly believe that the two vaccines currently available are both safe and effective. They have been developed and produced in record-breaking time, but scientists have been developing this vaccine technology for years. These vaccines have been studied on thousands of volunteers, and no major side effect issues have been identified. After reviewing the available data on both vaccines, I feel confident that the potential benefits far outweigh the risks. Both myself and my wife, Dr. Karlyn Armbruster, received the vaccines today (Monday) without hesitation,” said Armbruster.
Anyone with questions or concerns about the vaccine should contact their healthcare provider.
What You Should Know About the COVID-19 Vaccine
- Both Pfizer and Moderna indicated efficacy of 95%.
- The Pfizer vaccine is currently recommended for those 16 years of age and older. The Moderna vaccine is recommended for those 18 years of age and older.
- You need two doses of the currently available COVID-19 vaccine. A second shot is needed 21 days after the first Pfizer shot and 28 days after the first Moderna shot.
- The vaccine will not give you COVID-19. The vaccine does not use the live virus that causes COVID-19.
- You may have some side effects, which are normal signs that your body is building protection. Side effects can include pain or swelling at the injection site, fatigue, headache, fever (less common), and chills (less common). These should go away in 24-48 hours.
- Currently, the CDC recommends that vaccinated individuals continue wearing a mask around others. Experts need to understand more about the protection that COVID-19 vaccines provide before deciding to change recommendations on steps everyone should take to slow the spread of the virus that cause COVID-19.
- The vaccine will be provided to people at no cost.
“Over time, vaccines can end this pandemic and allow a return to normalcy. Unfortunately, that is going to take time. Until larger numbers of people have been vaccinated, we need to continue to be diligent about infection control measures. While we know that the vaccines prevent symptomatic disease, we do not know if they completely prevent our ability to spread the virus. For that reason, even vaccinated people should continue to wear masks, wash hands, and maintain appropriate distance,” said Armbruster.
Details about further availability of the vaccine will be released when they become available. In the meantime, individuals can obtain vaccine information on the following websites: www.cdc.gov, www.fda.gov, and www.health.state.mn.us.
“Ultimately, trust science,” said Armbruster. “Science did not start the pandemic, but it can end it. The full reports from the vaccine trials are available online. Read the information for yourself; do not look to social media.”